A novel coronavirus virus was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China in 2019. (Illustration/Alissa Eckert, MS; Dan Higgins, MAM)
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the third novel coronavirus known to infect humans. The largest known ribonucleic acid (RNA) virus, it is believed to be spread by respiratory droplets, much the same as influenza.1-5 At least one study suggested SARS-CoV-2 may remain viable for several hours in lab generated aerosols, leading experts to recommend airborne precautions during procedures known to generate aerosolized secretions.6 The resulting disease, referred to as COVID-19, has a presentation that is nothing short of peculiar. This article will discuss the prehospital management of COVID-19 respiratory symptoms.
A pneumonia case cluster first began November 17, 2019, in the city of Wuhan, Hubel Province, China. The disease rapidly became an epidemic in China and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020. Initial symptoms tend to be primarily respiratory in nature, consisting of fever, cough, shortness of breath, and infiltrates on chest x-rays that are often bilateral.7 Unfortunately, although there are some unique aspects of COVID-19 in more seriously ill patients and later in the course of even mild to moderate cases, none of the initial signs and symptoms reliably help to differentiate COVID-19 from other viral respiratory illnesses. Although high fever has consistently been a reliable initial symptom in other coronavirus outbreaks, recent analyses of COVID-19 cases suggest fever is often low grade (< 100.4℉) and initially present in only 44 percent of patients.8 Significant myalgias (muscle aches) have been reported in 35 percent of patients; loss of taste and/or smell in 34 percent of patients; and gastrointestinal symptoms (nausea, vomiting, diarrhea, or abdominal pain) in 18 percent, sometimes as the only initial presenting problem.9
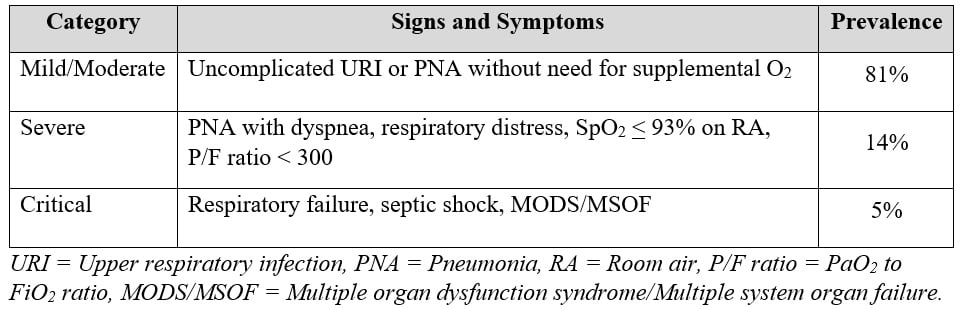
The spectrum of COVID-19 illness is extremely wide, ranging from mild to critical illness, categorized by the WHO10:
Critical care practitioners calculate a P/F ratio to express a patient’s degree of hypoxemia. The expression is the ratio of arterial to inspired oxygen (O2), which requires an arterial blood gas. Prehospital providers can make a similar calculation using a ratio of oximetry to inspired O2 (S/F ratio)11, where the O2 saturation (SpO2) is divided by the FiO2. A patient with a room air saturation of 85 percent (85 ÷ 0.21) would have a S/F ratio of 404. Although a P/F ratio of less than 300 suggests ARDS (Acute Respiratory Distress Syndrome), an S/F ratio of < 315 is suggestive of ARDS. The lower the ratio, the more significant the hypoxemia/ARDS.
The vast majority of COVID-19 patients will have only mild to moderate illness and are unlikely to be seen by emergency medical services (EMS) unless they have another medical or trauma related problem.10 Most patients recover at home within two weeks’ time.10 Chest radiographs (x-rays, CT scans) in COVID-19 patients demonstrate consolidation and ground-glass opacifications,12 sometimes even in asymptomatic patients. These films may be a more consistent COVID finding than PCR tests (reverse transcription polymerase chain reaction tests of nasopharyngeal swabbing).13
The primary trigger for a 911 response for COVID-19 symptoms will be difficulty breathing. Hopefully, your dispatch center follows Centers for Disease Control and Prevention and National Highway Traffic Safety Administration recommendations for alerting you when a known COVID positive patient resides at the call location and your prearrival information includes results of a vetted emerging infectious disease protocol advising you of a potential COVID-infected patient. Since scene safety is always your first priority, encourage the patient to meet you outdoors. When not possible and in safe neighborhoods, a single team member should enter the premises and assess signs/symptoms and exposures from a six-foot distance. When COVID signs and symptoms are present or there is a history of significant exposure, determine if the scene is unsafe, and immediately initiate source control by masking the patient and any bystanders. EMS personnel (only those absolutely needed) should don appropriate personal protective equipment. Under no circumstances should you place any EMS gear on any surface at the scene without a protective barrier such as a newspaper underneath it. Coughed or sneezed viral particles remain viable on surfaces for several days.6 Many services carry a COVID assessment plastic container with a thermometer, a blood pressure cuff and scope, an oximeter, and minimal other equipment needed inside a contaminated scene. These are easily decontaminated after the run.
One of the oddities of COVID-19 infections is hypoxia out of proportion to symptoms. Patients are sometimes referred to as “happily hypoxic.”14 As COVID-19 seems to be primarily a respiratory affliction, improving oxygenation improves outcomes. Optimally maintain O2 saturations between 90 and 96 percent. COVID-19 is one disease where your oximeter may be more valuable than your stethoscope. Lung sounds correlate poorly with the degree of distress; patients are rarely have reactive airways (wheezing) and are uncommonly hypercarbic.15-17 Initial assessment of O2 saturation on room air will inform you to the degree of hypoxia present. Following a baseline assessment, it is helpful to have the patient move about (if tolerated)—perhaps standing and walking to the stretcher or around the room—to assess for desaturation. In severe COVID-19 disease, desats can be profound and recovery, once at rest, can take quite some time. Target initial treatment at improving oxygenation, starting with a nasal cannula and escalating to a nonrebreather mask (if needed) to achieve as high of a saturation as possible.
In severe COVID-19 cases, ARDS may be present; hence, the value in calculating an S/F ratio. The ARDS seen in most COVID-19 patients is atypical; lung compliance is high (usually > 50 mL/cmH2O), meaning that the lungs expand easily. Breathing patterns are often significantly asynchronous, making it very difficult to manage patients who require mechanical ventilation. Patients often require very high minute volumes and, as mentioned previously, rarely become hypercarbic. In fact, most respiratory failure seen in COVID-19 patients is hypoxemic failure. Although O2 saturation is an excellent monitoring tool, many intensive care practitioners find that tachypnea may be more prognostic of impending respiratory failure; patients often seem to tolerate impressively low O2 saturations for long periods of time, seemingly without adverse consequences.17
Tools in the prehospital toolbox are not as robust as those in the hospital. Avoidance of aerosol generating procedures (AGPs) helps limit potential COVID-19 exposures. Nebulizers, suctioning, intubation, bag-valve-mask ventilation, and CPAP/BiPAP are all AGPs. There is little reason why a COVID patient would need a nebulizer, but CPAP could help with refractory hypoxemia, and ventilation/intubation might be needed for respiratory failure. In any of these AGP cases, add a viral filter to the respiratory circuit to trap exhaled secretions, which helps limit exposure of those in the immediate vicinity.15 Placing hospitalized patients in a prone position for 12-16 hours each day has been shown to improve oxygenation, likely because it shifts ventilation to better perfused areas of the lungs.15-16 Although quite impractical during EMS care and transport, outpatients with shortness of breath are often advised to try to sleep on their stomach.
If a patient remains acutely short of breath with low O2 saturations on a no-rebreather mask, a trial of CPAP might be helpful. Be thoughtful about this, however, as many O2-powered, disposable CPAP devices have a lower FiO2 than a nonrebreather mask. CPAP units entrain a significant atmospheric air into their circuits (venturi effect) to deliver the high gas flows needed to deliver CPAP. Use of some of these devices, particularly those powered with a flowmeter (as opposed to those connected to a 50 pounds per square inch high-pressure port) can result in a lower FiO2 being delivered than that from a nonrebreather. Adding a nasal cannula at 10-15 liters per minute under the CPAP mask might produce a FiO2 comparable to the nonrebreather. CPAP that uses a portable ventilator will allow the delivered FiO2 to be set at 1.0 (100 percent). As COVID-19 patients typically move high volumes of air and rarely retain CO2, BiPAP would not e helpful in this population.17
Care of the COVID-19 patient may require you to adjust your thresholds for O2 saturations. It is not uncommon to see saturations in the 60s and 70s, sometimes relatively well tolerated. Respiratory patterns can also be abnormal and well tolerated by the patient, as well. Tachypnea is common; COVID-19 patients often require high-minute volumes, so you can expect to see respiratory rates in the 30s. Acute deterioration in COVID-19 patients is often delayed, happening most commonly between days 7-9 of their illness.9 This is particularly important when considering treatment of in-place protocols in use by EMS, as COVID-19 patients who deteriorate will often “crash and burn” within a matter of minutes.
If you are treating a COVID-19 patient with respiratory distress and are only able to achieve an SpO2 of 87 percent on a nonrebreather mask, but the patient tells you he feels comfortable and you observe a respiratory rate in the mid-30s, it makes little sense to escalate therapy. Conversely, a patient who is barely conscious, with a respiratory rate in 50s and saturations in the 40s needs more than high-flow O2. You might trial CPAP. However, in all likelihood, you will need ventilatory assistance. Bag-valve-mask ventilation using a viral filter and intubation by the most skilled person on scene, preferable use of video laryngoscopy would be the safest intervention. Expect the need for high-dose sedation achieve synchrony with your ventilator.15 Ventilator modes also challenge clinicians in COVOD-19 patients because of their frequent asynchrony. Often, continuous mandatory or assist-control ventilation modes are best tolerated. On less sophisticated transport ventilators, pressure-regulated volume control ventilation may provide protection from high inspiratory pressures during transport. Anecdotally, and owing to the availability of additional hospital interventions such as high-flow nasal cannula, outcomes may be better for COVID-19 patients who are managed without intubation. It may be best for prehospital clinicians to use the least invasive means of addressing hypoxia, reserving intubation for cases where clinical condition clearly calls for escalation of treatment.
Two other EMS interventions are worth mentioning: antipyretics and fluids. Most EMS services carry antipyretics for pain/fever. Acetaminophen is the preferred antipyretic in COVID-19 patients. Although not contraindicated, there were some reports of younger patients early in the pandemic who received other nonsteroidal anti-inflammatory drugs and developed severe disease.16 Additionally, fluids worsen O2 saturations in COVID-19 patients. If you must administer intravenous fluids, use very conservative volumes.15,17
References
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270.
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020; 382:727.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565.
- Perlman S. Another Decade, Another Coronavirus. N Engl J Med 2020; 382:760.
- Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. National Science Review, , nwaa036, https://doi.org/10.1093/nsr/nwaa036.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med 2020; 382:1564-1567. doi:10.1056/NEJMc2004973.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med February 28, 2020. doi:10.1056/NEJMoa2002032
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395-497.
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected, Interim Guidance 13 March 2020. On-line: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed April 23, 2020.
- Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi:10.1378/chest.07-0617.
- Zhao W, Zhong Z, Xie X, et al. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol 2020;214(5):1072-1077. doi:10.2214/AJR.20.22976. Epub 2020 Mar 3.
- Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 Feb 26:200642. doi: 10.1148/radiol.2020200642. [Epub ahead of print].
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497.
- Anesi GL. Coronavirus disease 2019 (COVID-19): Critical care issues. In: UpToDate, Manaker S (Ed), UpToDate, Waltham, MA, 2020.
- Kim AY. Coronavirus disease 2019 (COVID-19): Management in adults. In: UpToDate, Hirsch MS (Ed), UpToDate, Waltham, MA, 2020.
- Cascella M, Rajnik M, Cuomo A, et al. Features, Evaluation and Treatment Coronavirus (COVID-19) [Updated 2020 Apr 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
Mike McEvoy, PhD, NRP, RN, CCRN, is the EMS coordinator for Saratoga County, New York, and the professional development coordinator for Clifton Park and Halfmoon Ambulance. He is a nurse clinician in the adult and pediatric cardiac surgery intensive care units at Albany Medical Center, where he also teaches critical care medicine. McEvoy is the chief medical officer and firefighter/paramedic for West Crescent Fire Department in Clifton, New York. He is also the chair of the EMS Section board of directors for the International Association of Fire Chiefs and a member of the New York State Governor’s EMS Advisory Council. He is a lead author of the textbook Critical Care Transport, the “Informed” Pocket References (Jones & Bartlett), and the American Academy of Pediatrics textbook Pediatric Education for Prehospital Professionals (PEPP).



Let me give it to you inlay terms. A person can be sitting down on a nasal cannula at 90 percent Oxygen saturation and when moved they might go to low seventies. It could take from 2 to 10 minutes to get back to 90 percent. The patient position can have a lot to do with the oxygen saturation. At first when dealing with these patients it was scary when they were in the low 70s but after a few weeks it became a new normal. While you do not want them staying in the seventies even if they tell… Read more »