As someone who began his practice as a paramedic before pulse oximeters came on the market, I have a real appreciation for the technology. It turns out that blue lips are not a particularly precise indicator of hypoxia. Oxygen saturation is not only a standard vital sign but also gives us crucial information about our patients.
If only the device wasn’t so finicky. We all know that things like cool extremities, poor perfusion, excess light, fingernail polish, acrylic, nails, excessive movement, and even dark skin can produce an inaccurate result. This can be highly frustrating and detrimental to patient care. Having. a “toolbox” to overcome these things would be helpful.
Pulse oximetry works by placing a probe on the skin that produces a red light and an infrared light. The light passes through the tissue and is captured by a sensor on the other side of the tissue. The change in color is interpreted by a very small person inside the machine who does math and produces a number that reflects the percentage of hemoglobin that is bound with oxygen molecules. Or maybe it’s a microprocessor that does that.
At any rate, the probe needs both adequate blood flow in the area and an adequate path to send out and receive the light signal. This is called the transmittance method, which is what devices in the EMS industry use. There’s another method called the reflectance method that we will get into a little later.
The most commonly used sensor is the reusable finger probe. It works great 80% of the time (a statistic I just made up). Some of the patients that fall in the other 20%, though, are really sick patients, and we need an accurate SpO2 reading.
Looking at the waveform, in addition to the number, is an important step. You can’t rely on the number if you don’t have a consistent waveform. So, what are some tips to try and capture a good waveform?
The first one that we typically use is a good one, which is trying a different finger, or fingers on the other hand. What if our patient has painted fingernails? Sometimes that matters, and sometimes it doesn’t. Darker nails are more problematic than lighter nails, and many services carry little packages of nail polish remover. Removing the nail polish is a good next step. What about acrylic nails?
I had a patient recently with black acrylic nails who was in significant respiratory distress, and I couldn’t get a good signal. I assumed she would object to me ripping off one of her acrylic nails, but I really needed to know her SpO2. That call sent me down the rabbit hole that produced this article.
First of all, although the manufacturers factor in the fingernails, there’s not much that is magical about the light beam going through the nail and being read on the other side. Turning the probe sideways could overcome the problem of trying to pass the light beam through an obstructive fingernail.1 Remember, the light beam just has to pass through a relatively thin amount of tissue and be captured on the other side. Try that approach and observe for a good waveform.
If you think there is poor capture due to excessive light, cover the hand with a blanket. This is also helpful if the patient has cold hands, and rubbing the area and/or adding a heat pack isn’t a bad idea. You may experience poor capture due to movement, particularly during transport. Try to secure the probe’s cable and not leave it dangling.
Do you want to know what doesn’t work? Putting the finger probe on the earlobe. I know it’s tempting, and it makes sense because the earlobe is an easily accessible bit of perfusing tissue. I’ve seen folks do this occasionally, both in the field and in the hospital. But I’m sorry to tell you that the technique has been studied2,3 and the evidence shows it produces inaccurate results. Be an evidence-driven provider, and don’t do it. The concave shape of the finger probe is just not conformed to work on the ear.
Have you ever tried placing a finger probe on a patient’s toes? Me neither, but I’ve heard it suggested. If the perfusion in the foot is adequate, it might work because toes are just short, stubby fingers, kind of. I’ll try it on myself tomorrow at work, and I’ll let you know what I find out. Next day: results were disappointing. Plus toes are gross and if you try it, really decon the probe afterward. Please. The exception, of course, is neonates and infants, where the adhesive probes (that I hope you carry on your ambulance) usually work well.
What do you do if those attempts at troubleshooting fail? Particularly if you have a really sick patient and an accurate SpO2 is a critical diagnostic tool? Let’s go back to talking about the ear. The ear is a go-to spot for a patient with poor perfusion. The blood flow to the ear is robust. Plus, the ear is stable and shouldn’t be moving around a whole lot. While the finger probe doesn’t work on the ear, the sensors that are designed for the ear work well.
There are two types of ear probes currently on the market. One is a reusable clip that goes on the earlobe. In my opinion, it’s pretty good, but what’s better is the “disposable” ear probe that is designed to go in the ear concha (see Figure 1).
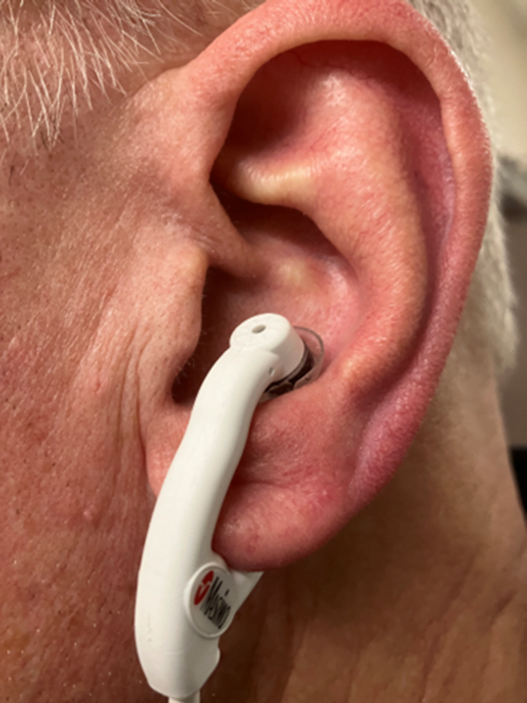
Figure 1: Masimo E1 Disposable Ear Concha Sensor. Photo courtesy of author
They work great. They cost around $35, which is why I put quotes around the word “disposable” above. Not that I would advise you to decon one after use and put it back in your kit. I don’t think the editors would let me suggest that in writing. But I will say that this should be an item stashed on every monitor capable of capturing SpO2. The ear concha probe works on the Zoll X-Series, Stryker/Physio-Control LIFEPAK 15 and Phillips Tempus monitors.4,5,6 The Philips MRX has an option for a proprietary reusable ear clip sensor.7 If your service doesn’t currently carry ear sensors, please advocate that they purchase them.
Medical device manufacturers have been developing other probes to overcome low perfusion states. One probe that is used in the hospital is a nasal alar probe (see Figure 2).
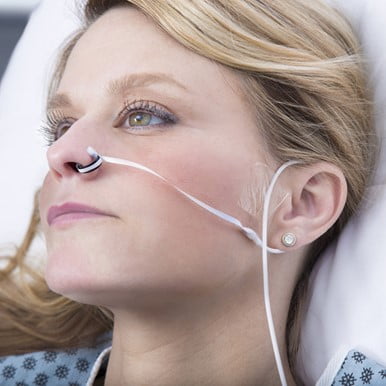
Figure 2: Philips Nasal Alar Sensor. Photo courtesy of Philips Corp.
The alar is the soft, fleshy part on the lateral aspect of the nostril. This area has a rich vascular bed that is supplied by the carotid artery. It is highly reliable even in low perfusion. As of this writing, however, none of the EMS monitors supports the use of a nasal alar sensor, which is unfortunate.
I speculated that the ear concha probe discussed earlier might work in the nasal alar area. I conducted a study utilizing a reasonably healthy adult male and found a correlation between the standard finger probe with a good waveform and the ear concha probe placed in the nasal alar (see Figure 3).
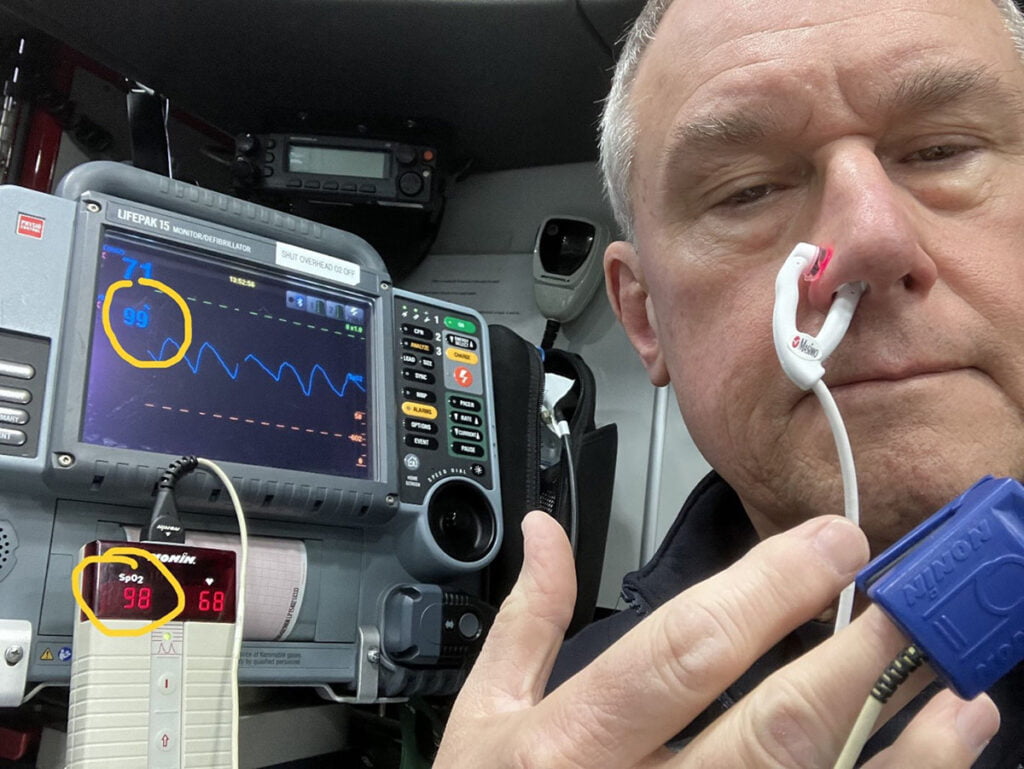
Figure 3: Comparison of finger sensor to E1 ear concha sensor placed in the alar area. Photo courtesy of author
This study had no IRB approval, had a tiny sample size (one), and was unblinded, so the results are likely somewhat biased. I think it would be a good topic for future study.
Would it be inconsistent of me to suggest you try this as a last-ditch effort if you encounter a really sick patient who is not giving you a decent pulse oximetry reading? I just cautioned you not to use the “off-label” application of the finger probe on the ear. Listen, you’re the professional; it’s your toolbox. Anyway, it would be nice if the manufacturers would make this an option. But don’t hold your breath (no pun intended). Right now, Philips is the only monitoring device manufacturer that enables the use of a nasal alar probe, but not for their EMS monitors.
The last probe I’d like to discuss is a forehead probe (see Figure 4).
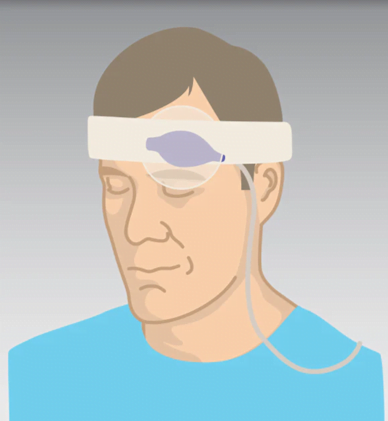
Figure 4: Forehead Reflectance-type Sensor.
Photo courtesy of Cables and Sensors, LLC.
This is another device designed to overcome low perfusion in the extremities. It works in a different manner from typical SpO2 probes. Instead of a direct capture of the light signal through tissue (transmittance method), it works through the reflectance method, which means the optical signal passes through tissue and is received indirectly. It goes on the forehead above the eyebrows, another highly vascular area less vulnerable to poor perfusion.
Masimo markets two forehead devices, one reusable and one disposable. The disposable probes run around $35, and both types require a headband (which is reusable). I’m unsure how practical this probe would be for field use, but perhaps for critical care transport? The technical representatives I spoke with from the three monitor companies tell me that their EMS monitors do not support the forehead probes at this time.
The technical rep from Masimo, however, told me there’s no reason they shouldn’t work. This individual speculated that the companies didn’t test it on their monitors to give their stamp of approval. The little guy who lives in the machine should be able to do the calculations. A monitor rep said that testing of different sensors was based on the demand from the market. There has not been any demand from the EMS market for this style of sensor. Yet.
You’re no doubt already aware that patients with carbon monoxide poisoning will exhibit SpO2 readings that you can’t trust. You may also be aware that pulse oximetry readings may be inaccurate on persons with darker skin.8 Unfortunately, there aren’t any tips to overcome this discrepancy; that is something the industry is working on. Let’s hope the engineers come up with a solution soon.
In summary, you should have troubleshooting tips and tools to use when encountering a very sick patient but you’re having trouble getting the essential SpO2 reading. Know what isn’t going to work, such as putting a finger probe on the earlobe or an adhesive sensor on the bridge of the nose. Whatever method you try, use the waveform to inform you whether you can trust the number. One final tip is to read the pulse oximetry chapter in your device’s user manual. The manuals are easy to find online and are free.
References
- Klaus D. Torp; Pranav Modi; Elizabeth J. Pollard; Leslie V. Simon. Pulse Oximetry. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470348/
- Haynes, J. M. The ear as an alternative site for a pulse oximeter finger clip sensor. Respiratory Care, 2007; 52(6), 727-729. https://rc.rcjournal.com/content/52/6/727/tab-pdf
- Hlavin D, Varty M. Improving Patient Safety by Increasing Staff Knowledge of Evidence-Based Pulse Oximetry Practices. Crit Care Nurse 1 December 2022; 42 (6): e1–e6. doi: https://doi.org/10.4037/ccn2022998
- Zoll X Series Advanced Operator’s Guide, Revision D, Chapter 10 Pulse CO Oximetry (SpO2) [Internet]. 2021 Oct [cited 2024 Mar 14]. Available from: https://www.zoll.com/-/media/product-manuals/x-series-advanced/05/9650-005355-05-sf_d.ashx
- Stryker Physio Control LIFEPAK 15 Monitor/Defibrillator Operating Instructions. Chapter 4 Monitoring: Monitoring SpO2, SpCO and SpMet [Internet]. 2019 Jan [cited 2024 Mar 14]. Pages 69-79. Available from: https://www.stryker.com/content/dam/stryker/ems/resources/operating-instructions/international/3314911-030_int-eng_lifepak_15_operating_instructions.pdf
- Philips Tempus Pro User/Operator Manual [Internet]. 2022 Sep [cited 2024 Mar 14]. Page 223. Available from: https://www.documents.philips.com/assets/Instruction%20for%20Use/20210913/f810edd239df41c19c96ada200c9eb6d.pdf?feed=ifu_docs_feed&_ga=2.57488418.921387667.1710511275-1668671750.1699622043
- Philips HeartStart MRx: Instructions for Use. Chapter 8: Pulse Oximetry [Internet]. 2016 [cited 2024 Mar 14]. Pages 99-106. Available from: https://images.philips.com/is/content/PhilipsConsumer/Campaigns/HC20140401_DG/Documents/HC20200428-MRx-L-IFU.pdf
- Cabanas AM, Fuentes-Guajardo M, Latorre K, León D, Martín-Escudero P. Skin Pigmentation Influence on Pulse Oximetry Accuracy: A Systematic Review and Bibliometric Analysis. Sensors 2022 Apr 29;22(9):3402. doi: 10.3390/s22093402. PMID: 35591092; PMCID: PMC9102088.
Clay Odell, BS, NRP, RN, is a paramedic-firefighter with Newport (NH) Fire-EMS, and a paramedic for New London Hospital EMS. He is a past state EMS bureau chief, state trauma system coordinator, EMS service chief, and a flight crew member at Dartmouth Hitchcock Advanced Response Team. Clay has been a paramedic since 1985, and a registered nurse since 1997.


What about capnography?
Great if you buy these special probes. Not very field applicable in my opinion.