
On the go? Listen to the article in the player below!
It’s a cold Friday night in January. You just finished an uninterrupted meal at the local Chinese restaurant with your partner when you’re dispatched to a house in town for an 88-year-old male with trouble breathing. You pull up to a one-story house, sitting slightly up on a grade. The lights are on and the front door is open. As you enter a musty smell catches you as you lock your eyes on an older male. He’s tri-poding in an old, worn, puffy rocking chair. It’s clear from his fast, shallow, pursed-lip breathing he’s in distress. You introduce yourself and accept his outstretched hand for a shake.
His skin is cool and moist, his radial pulse fast and irregular. He seems to be looking straight past you, focusing on the wall. Each word he speaks is an immense effort. You ask his name and he gasps “Charles…Winston.” You start asking Mr. Winston yes and no questions to ease his work of responses as your partner is getting him hooked up to the monitor. What assessment findings are important, what are you considering at this point?
Let’s break down some of the things that should jump out at you:
Patient Positioning – You recognize tri-poding; Mr. Winston is trying to streamline his airway for ease and efficiency. He is also trying to open up his thorax to maximize tidal volume.
Pursed Lips – What do you think about the dynamics of airflow? You see a person trying to exhale slowly through a smaller hole made by pursing their lips; Why? This helps prolong the expiratory phase and increase volume in the lower airways, inhibiting alveolar collapse during full exhalation. Mr. Winston is trying to create PEEP to help keep his alveoli wide open and allow for more gas exchange.
Sentence Structure – Why is Mr. Winston so taxed and only able to say one or two words? Why doesn’t he have the ability to converse like he does every other day? He is relaying on compensatory mechanism. He is so dependent on airway positioning, accessory muscle use, and support that he can’t change that up to speak. He has to maintain his fast respiratory rate and talking would interrupt his compensation.
Skin, Color, and Temperature– Just remember you can’t fake pale/cool/diaphoretic. When the body is in shock, noradrenaline helps to shunt blood to the core maintaining circulatory volume in the thorax and maximizing cardiac output.
Your eyes now gaze down to your monitor as your partner is trying to point out a couple of values. What below most concerns you?
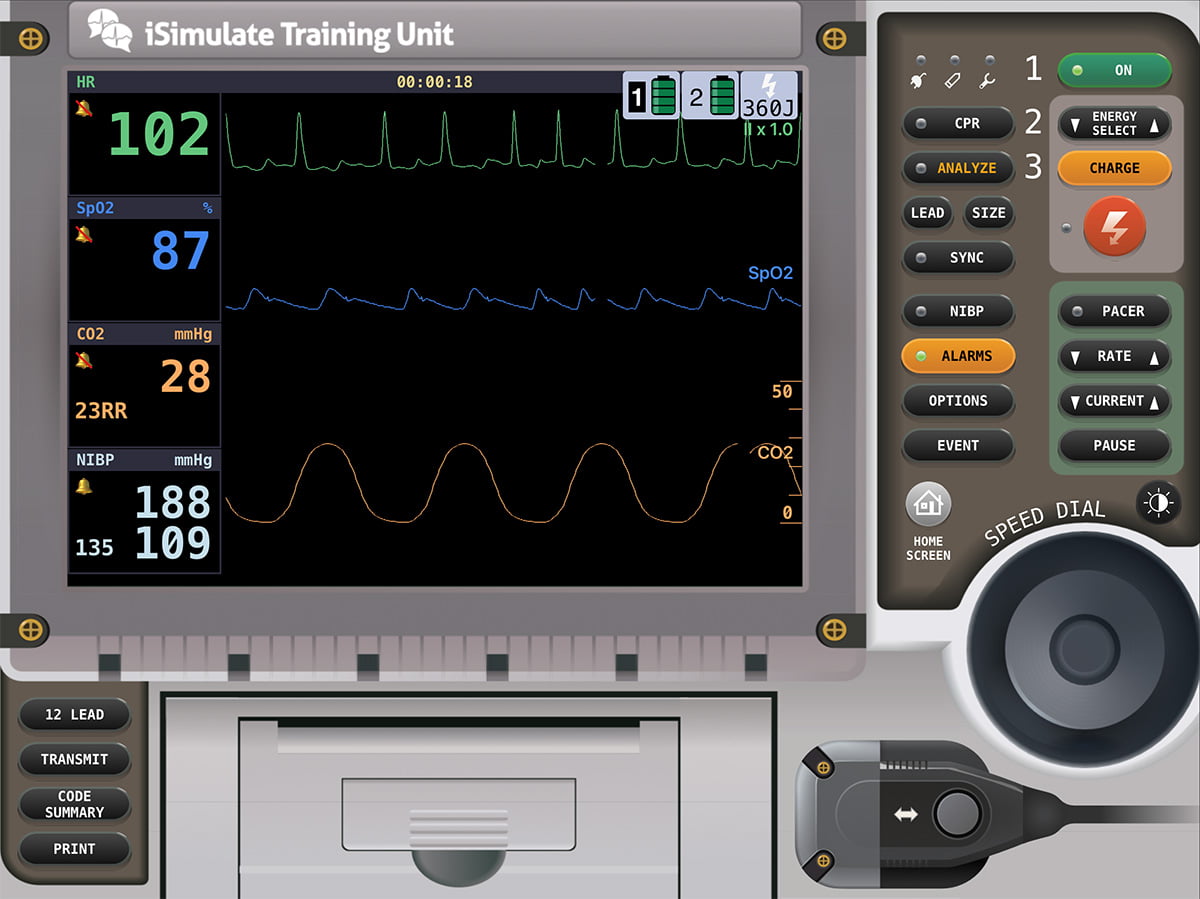
Your eyes go right to the pulse oximeter reading. Our training has instilled a robotic tendency to walk into the room and connect the pulse oximeter probe to the digit farthest from the heart and simultaneously cut off circulation to the same digit with a blood pressure cuff. In a certification test, this robotic technique would gain maximum points, but why? What feedback are you getting out of that? What actionable information come from blood pressure and a pulse ox? Are there other diagnostics with greater value in time-sensitive patient care?
In this instance, we should be alarmed by the rounded wave, fast rate, and low value of the EtCO2 (end-tidal CO2). The rounded (dampened) wave can correlate to fluid in the alveoli. The fast rate and low EtCO2 is consistent with pulmonary edema occurring in a state of low alveolar capillary perfusion and tachypnea. You also correlate tachypnea from the stressed state and low SPO2 as symptoms of poor gas exchange. You then move to auscultate lung sounds. After lifting his shirt and placing your stethoscope on the skin, you hear that crunchy, crackly sound that you know all too well to be fluid confirming your suspicions.
Auscultate
You continue to assess the monitor and notice an irregular, fast heart rate with a low SPO2 value; what are your differentials and treatments?
Let’s revisit our assessment findings. Mr. Winston was tachypneic and tripoding with pursed lips. We realized earlier he was trying to create PEEP to keep his alveoli balloons inflated. This helps to maximize the surface area available for oxygen exchange of an otherwise flooded balloon. Remember, oxygen doesn’t diffuse as well through liquid as CO2 does. The alveoli need more area free from contact with edema to allow oxygen to diffuse into the capillaries.
Should we support that? How do we support that?
We can place them on CPAP (continuous positive airway pressure). This is a simple device that uses a little valve on a spring under compression to restrict full exhalation.

This device prevents the complete exhalation during the patient’s respiratory cycle, thus retaining some of what would otherwise have been exhaled gas to help maintain alveolar ballooning. By doing so, the alveoli stay inflated, increasing the amount of surface area not in contact with the pulmonary edema. The failure of the left ventricle causes blood to back up in the pulmonary circulation, increasing pressure in the pulmonary capillaries. This increased pressure causes plasma to leak out of the capillaries into the areas surrounding the alveoli and eventually into the alveoli. Increasing airway pressure through volume retention balloons the alveoli and increases surface area that isn’t in contact with edema and allows for oxygen exchange. You start Mr. Winston on 5 cm of H2O of PEEP and you see the following vital signs on your monitor change. Why did this happen? Is this concerning?
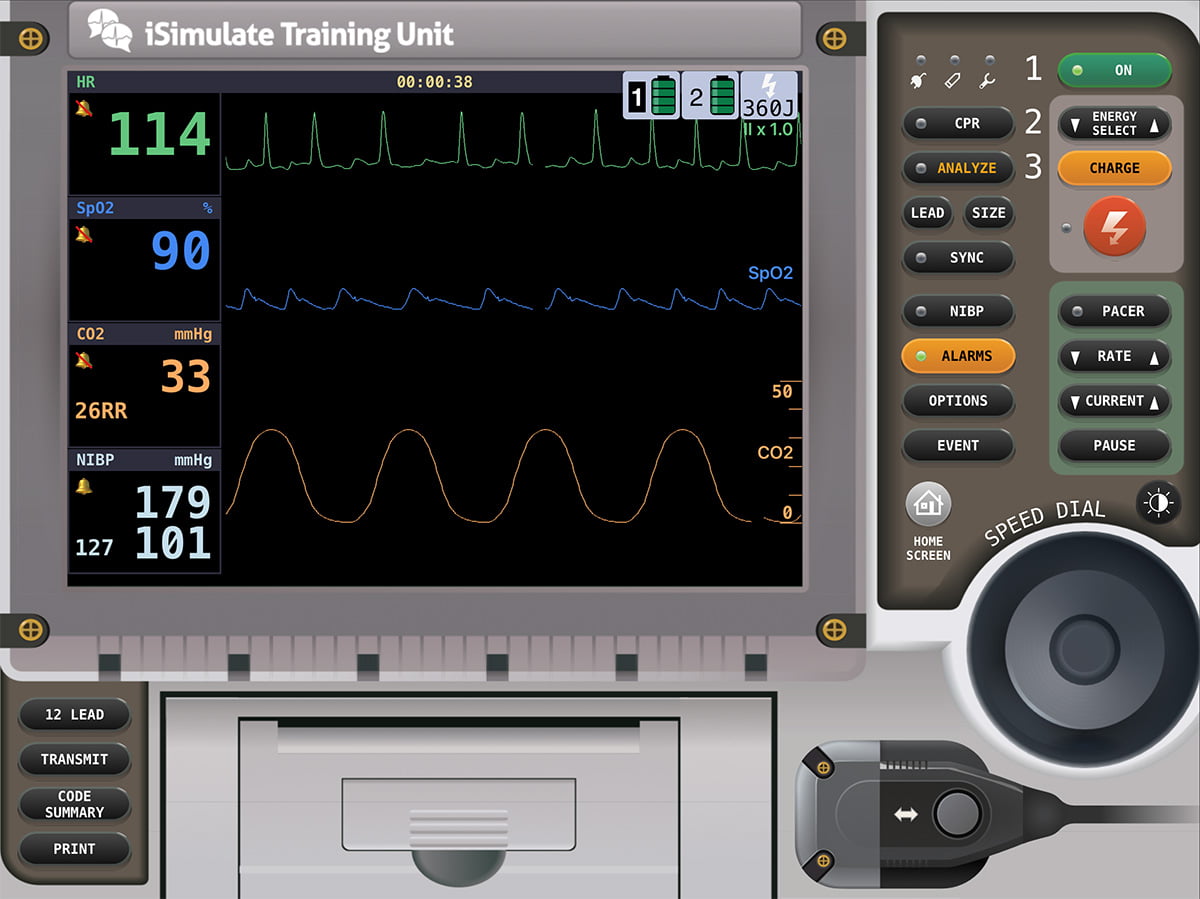
The increase in EtCO2 is an expected side effect of added PEEP. By restricting full exhalation with the added PEEP more CO2 accumulates in the airways. This is an accepted change with your treatment; You know increased pressures from CPAP will cause alveolar ballooning and improve oxygenation. The increased pressures in the thorax remind you to check the blood pressure. Increasing the ballooning of the alveoli be retaining pressure in the thorax could squeezing the lower pressure vessels including the vena cava or even the right ventricle. It is this pinching or “tension” on the vena cava that can cause a decrease in preload, the blood return back to the heart. This concern for intra-thoracic pressure change is the reason most protocols require a blood pressure minimum of 90 or 100 for CPAP application.
Are you satisfied with the condition of your patient on 5 cmH2O of PEEP, or do you need more? How do you evaluate the need for more?
Mr. Winston seems to be tolerating the CPAP. He’s not grabbing at the mask; he still can only speak in one to two-word sentences. His respiratory rate elevated and his work breathing is increased. He has to stay sitting in a tripod position and gets flustered even with a short move from the position for you to apply ECG electrodes. You acquire a 12 lead/15 lead EKG to evaluate ST-segment changes and look for signs of an MI that could be the trigger for this acute event.
After consulting with your partner, you opt to increase the PEEP to 7.5cmH2O and administer nitroglycerin to reduce the preload. You cross-check the nitroglycerin and administer it. In congestive heart failure (CHF), the patient has an enlarged left ventricle which reduces the amount of blood it can eject. Compared to the ejection from the right ventricle, there is a mismatch. This mismatch causes a backup as the outflow from the LV can’t keep up with the inflow from what the RV is sending. This mismatch creates higher pressure in the pulmonary capillary bed that wraps around the alveoli and flows through the lungs.
The capillaries stretch under pressure and leak plasma and even red blood cells from the blood into the alveoli. It is this plasma mixed with the air from breathing that creates the “bubbling” or “pink frothy sputum” and crackles. While PEEP helps with oxygenation by increasing alveolar surface area, nitrates to help reduce the pressure in the capillaries. Vasodilation reduces blood flow back to the RV and reduces LV workload. The low EtCO2 level you saw on the monitor originally is from a reduction in the return of CO2 to the lungs and increased respiratory rate blowing off CO2. The stressed state, arterial vasoconstriction, and low cardiac output due to cariogenic shock impaired the perfusion and CO2 flow to the lungs from the tissues.
You now find these vital signs a minute later.
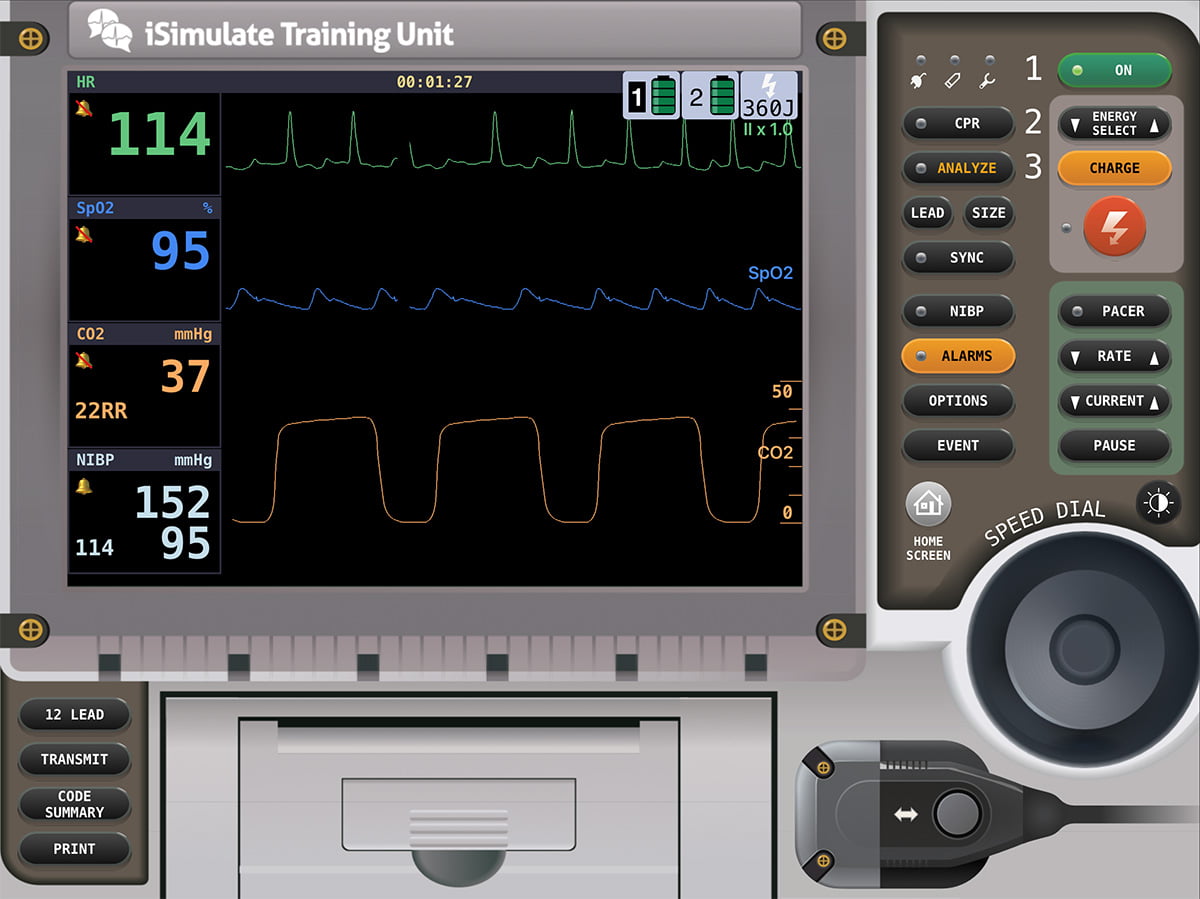
Mr. Winston is starting to speak in four-to-five word sentences, and he is starting to look around the room. You see the beginning of a smile underneath the mask, and he grabs you with his hand thanking you with a firm shake. You’re now able to obtain a history from him, as well as find out where he was born and raised and how long he’s lived in town. He tells you he has had heart problems since his heart attack 15 years ago. He has left-sided heart failure and blood pressure control problems along with type two diabetes. He takes a water pill, beta-blocker, and oral diabetic medication. He was born a farm boy Iowa and worked on the farm until he moved out to your neck of the woods to be with his grandchildren who live down the street.
Looking back at this patient, what was wrong?
It all started 15 years ago with a myocardial infarction that damaged and weakened the muscle of his left ventricle. This weakening over the years has required the heart muscle to work harder and caused ventricular remodeling and dilation. The muscle is weaker and doesn’t stretch well causing weaker contractions and poor cardiac output and retention of blood in the ventricle. Now, he has reduced chamber function on the left side at baseline and can’t compensate for stress. When acute stressful events happen, he has to vasoconstrict and increase vascular resistance to maintain perfusion.
He gets flooding of his heart, but with the left ventricle already backed up and unable to empty, that acute flooding backups up into the lungs commonly called flash pulmonary edema. How do we combat this acute emergency? Our goal when battling fluid in the lungs is to maximize the oxygen exchange surface area. We accomplish this through PEEP. Adding this controlled and expected reduced exhalation it allows the alveoli to stay ballooned. This, in turn, increases the amount of area not in contact with fluid letting oxygen exchange more easily. As a reminder, oxygen does not diffuse through liquid well; carbon dioxide does. It diffuses about 20 times higher than oxygen. This is why capnography is a valuable tool for measuring ventilation in the presence of pulmonary edema or drowning patients who have fluid in their lungs; measuring the end-tidal CO2 value gives us a measure of ventilation even when oxygen exchange is impaired and Sp02 values are impaired.
We also use nitrates to help dilate vessels and reduce the blood return to the heart, thus reducing the amount of that fluid overload (flooding). We’ll also need to check to ensure that it’s not an acute heart attack that triggered this event.
References
- Mieloszyk, R. J., Verghese, G. C., Deitch, K., Cooney, B., Khalid, A., Mirre-Gonzalez, M. A., Heldt, T., & Krauss, B. S. (2014). Automated quantitative analysis of CAPNOGRAM shape for COPD–normal and COPD–CHF classification. IEEE Transactions on Biomedical Engineering, 61(12), 2882–2890. https://doi.org/10.1109/tbme.2014.2332954
- Caroline, N. L., Elling, B., & Aehlert, B. (2022). Nancy Caroline’s emergency care in the streets. Jones & Bartlett Learning.
- Carbon Dioxide Gas Transport. Carbon Dioxide Gas Transport Charles L. (n.d.). Retrieved December 10, 2022, from https://www.meddean.luc.edu/lumen/meded/medicine/pulmonar/physio/pf4.htm
Chris Kroboth has been a career paramedic/firefighter for over 17 years and in EMS for over 23. He has been in prehospital and in-hospital education for the past 18 years. His last assignment before returning to operations was as the EMS training captain in charge of continuing education programs and certification. He is also affiliate faculty with the Virginia Commonwealth University Paramedic Program. He is the U.S. clinical education manager for iSimulate and also facilitates national conference clinical challenges to include EMS World, ENA and NTI.


