On the go? Listen to the article in the player below!
Clinical Case
Paramedics in a rural setting respond to a young man who is unresponsive, barely breathing, has pinpoint pupils and has fresh needle marks along his arm veins. Recognizing this as an opiate overdose they assist ventilations and attempt to administer IV naloxone, but have difficulty starting an IV line. They heard about nasal naloxone, so they squirt a vial of naloxone into the patient’s nose with a syringe (1 ml of 0.4 mg). It does not have any effect but they manage to establish an IV and administer another 0.4 mg IV. The patient awakens. Based on this experience, they decide nasal naloxone does not work and resist adoption of the concept during future discussions.
This case, and others like it, play out in EMS systems and hospitals throughout the country and the world. Well-meaning clinicians hear about nasal medication delivery and try it on a patient using an inadequate dose or poor administration technique. They decide it is ineffective and resist adoption of the concept into their practice. While nasal medication delivery won’t replace other effective methods of drug delivery, it has advantages that make it a useful tool in the right clinical situation. Understanding its benefits and limitations are important to proper utilization. Key concepts to understanding nasal drug delivery are: First pass metabolism; the nose brain pathway; bioavailability; and the therapeutic window. This article will discuss these concepts and should provide the reader with a deeper understanding of intranasal drug delivery so they can use it more effectively in their practice.
First-Pass Metabolism: Effect on Drug Levels and Onset of Therapeutic Effect (See Figure 1)
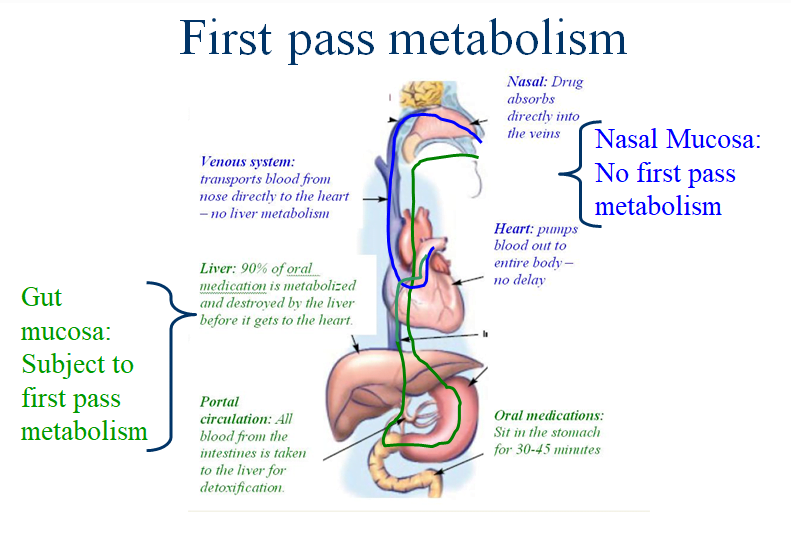
When a medication is swallowed (oral or p.o. meds) it is absorbed through the gut mucosa and enters into the “portal” venous circulation. The portal blood then flows into the liver where enzymes breakdown the vast majority of the drug. This destruction by the liver is called hepatic first pass metabolism and results in 90-95% of the drug being removed from the circulation. It also leads to delays of 30-60 minutes before any clinical effect is noted. Because of first pass metabolism, oral drug doses need to be much higher than other routes of administration to ensure a therapeutic drug level is achieved. Nasal drugs are absorbed directly into the venous circulation of the head and neck and do not undergo first pass metabolism. This leads to a rapid clinical effect (a few minutes) using doses much lower than oral drugs.
Nose Brain Pathway: (See Figure 2)
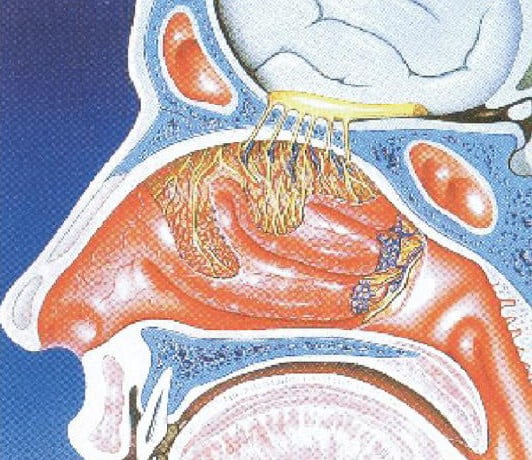
The upper aspects of the nasal cavity are covered with nerve endings that provide our sense of smell (olfaction). This area is called the olfactory mucosa. When medications come into contact with the olfactory mucosa they can be quickly transported up the nerve fibers into the brain and cerebral spinal fluid (CSF). This is called the nose brain pathway and partially explains why centrally acting drugs such as naloxone, pain medications, sedatives and anti-seizure medications can exert effects rapidly on the central nervous system.1
Bioavailability
Bioavailability is defined as the percentage of an administered drug that ends up in the blood stream. By definition, any drug administered through an IV is 100% bioavailable. As discussed in the first pass metabolism paragraph above, most drugs given by mouth are destroyed by the liver so only 5%-10% is bioavailable and at a delayed time period. Research has demonstrated that many drugs are not bioavailable via the nasal route, so only selected drugs can be used in this fashion. The absorption of these drugs (their bioavailability) is influenced by many factors, some which can be influenced by the paramedic and are discussed below. Regardless, no nasal drug is 100% bioavailable and all have a few minutes delay in absorption so the doses are almost never the same doses as an IV medication. Giving the wrong dose (too little) will guarantee failure to achieve desired clinical effect (this is research dependent for each medication but in general at least twice the IV dose is required).
Bioavailability Factors That Can Be Impacted by the Paramedic
Minimize Drug volume: The nasal cavity has limited capacity to hold medications for absorption. If the volume is too large or just poured down the nose, the excess fluid simply runs into the mouth and is either spit out (0% bioavailable) or swallowed and undergoes first pass metabolism. The exact volume of medication that is ideal for nasal drug delivery is not well defined. Volumes as low as 0.1 ml and as high as 0.5 to 1 ml per nostril are well studied and effective in clinical studies, whereas volumes in excess of 1 ml per nostril should probably be avoided, or divided and given over several doses.2, 3
Maximize Drug Concentration: Directly related to the volume issue, the drugs concentration is critical. In general the most concentrated form of the drug should be used for nasal delivery to minimize the volume of drug delivered.2, 4
Maximize absorptive surface area by using both nasal cavities: Since there are two nasal cavities it makes sense to use as much surface area as possible to absorb the medication, especially if the drug is fairly dilute. By using both nostrils you will double the surface area and halve the volume of drug administered per nostril.4 This is not necessary in highly concentrated drugs where you are only giving a tiny volume (0.1 ml) as you could lose more drug to transferring nostrils than you would gain by increasing absorptive surface.
Use a delivery system that maximizes mucosal coverage by creating an atomized spray: Prior to the advent of nasal atomizers, nasal drug delivery efficacy was unpredictable likely due to issues of patient cooperation and runoff of the liquid straight into the throat. Atomization devices that can deliver an exact dose of fine particles have eliminated these two issues and markedly improve the clinicians’ ability to rapidly deliver a medication broadly across the absorptive nasal mucosa, including up onto the olfactory mucosa. However, proper operation of the atomizer is crucial as the syringe driven models require a brisk compression to actually atomize the drug. Inadequate compression result streaming of the drug which defeats the purpose. Worries about patient injury from brisk compression are unfounded as the exit holes in these devices are so tiny that they prevent any high pressure from leaving the device.
Direct the atomized spray toward the top of the ear on the same side: Aiming upward and slightly lateral (toward the top of the ear) causes the medication to be directed away from the nasal septum (poorly vascularized) and towards the rich vascular mucosa of the turbinates and olfactory mucosa. This leads to better surface area coverage of the vascular mucosa and less runoff down along the septum into the throat.
Titration: As with IV medications, nasal medications have a fast enough onset that if the clinical effect is not achieved in a reasonable time frame, repeat dosing can be administered.5
Be aware of nasal mucosal abnormalities: While a small amount of nasal discharge does not affect nasal drug absorption, there is evidence that vasoconstrictor use (Afrin- oxymetazoline) does.6 Suctioning the nasal passage to remove foreign material prior to drug administration may be appropriate though it has not been studied. Being aware of the patients nasal mucosal health will help you recognize situations where nasal drug delivery might not be ideal allowing you to make other plans if necessary.
Therapeutic Window and Safety of Intranasal Medication Delivery: (See Figure 3)
The therapeutic window is the drug level in the blood that produces a clinical response (minimum effective concentration) without causing any serious adverse effects (minimum toxic concentration). For example, if you administer an opiate pain medication, your minimal effective concentration is that level where good pain control is achieved, while your minimum toxic concentration is that level where significant sedation and/or respiratory depression starts. This concept has direct applicability to the safety of nasal drug delivery compared to IV drug delivery as demonstrated in Figure 3.
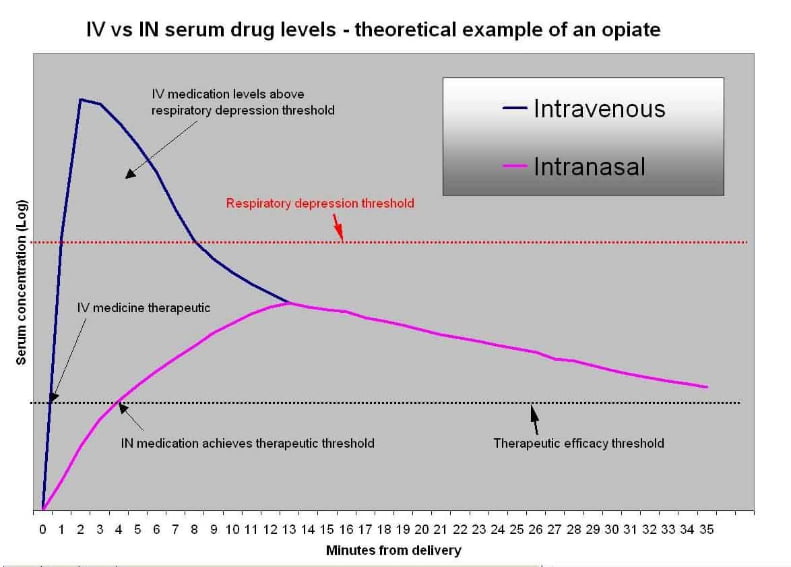
Figure 3: Therapeutic Window and Safety – IV Versus Nasal opiate
This diagram visually demonstrates the safety differences between an intravenous and nasal opiate given for a painful condition. The IV drug peaks immediately and often surpasses the minimum toxic concentration leading to respiratory depression for the first 5-10 minutes. Nasal drugs, in contrast, absorb over many minutes and do not peak immediately.7 For that reason they rarely approach the minimum toxic concentration and rarely lead to respiratory depression. This provides a margin of safety. It also should make the clinician more comfortable with the higher doses required when giving a medication nasally. (Failure to give an adequate dose will simply result in failure to achieve therapeutic levels of the medication.) Interestingly, despite a slightly slower achievement of the minimum effective concentration, nasal medications achieve adequate pain control faster than IV medications in clinical settings primarily due to the delays required to start and IV line.8
Summary
Intranasal drug delivery is an established and well-studied method of delivering medications to properly selected patients. Its efficacy can be optimized by using an atomizer to deliver small volumes of concentrated medications in adequate doses (usually higher dose than IV, lower then oral). Used properly this tool can save you time, reduce needle stick risks, improve patient satisfaction (no needle) and potentially improve safety. Future articles will discuss clinical situations where you may find intranasal medication delivery useful to your practice.
References
- Wang, Z., et al., Nose-to-Brain Delivery. J Pharmacol Exp Ther, 2019. 370(3): p. 593-601.
- Mygind, N. and S. Vesterhauge, Aerosol distribution in the nose. Rhinology, 1978. 16(2): p. 79-88.
- Tsze, D.S., et al., Optimal Volume of Administration of Intranasal Midazolam in Children: A Randomized Clinical Trial. Ann Emerg Med, 2017. 69(5): p. 600-609.
- Dale, O., R. Hjortkjaer, and E.D. Kharasch, Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand, 2002. 46(7): p. 759-70.
- Karlsen, A.P., et al., Safety of intranasal fentanyl in the out-of-hospital setting: a prospective observational study. Ann Emerg Med, 2014. 63(6): p. 699-703.
- Shyu, W.C., et al., The absolute bioavailability of transnasal butorphanol in patients experiencing rhinitis. Eur J Clin Pharmacol, 1993. 45(6): p. 559-62.
- Malinovsky, J.M., et al., Plasma concentrations of midazolam after i.v., nasal or rectal administration in children. Br J Anaesth, 1993. 70(6): p. 617-20.
- Borland, M.L., L.J. Clark, and A. Esson, Comparative review of the clinical use of intranasal fentanyl versus morphine in a paediatric emergency department. Emerg Med Australas, 2008. 20(6): p. 515-20.
Timothy R. Wolfe, MD, is an emergency medicine physician in Utah. He received his medical degree from University of Utah School of Medicine in 1988.



Great article! well presented and detailed.